Learning Outcomes
i. Master the art of sketching an electrolytic cell, accurately representing its components and their arrangement.
ii. Identify and label the cathode and anode, the essential electrodes involved in electrolytic reactions.
iii. Understand the purpose of the electrolyte and salt bridge in facilitating electron flow and maintaining electrical neutrality.
iv. Explain the role of an external electrical energy source in driving electrolytic reactions.
Introduction
In the realm of chemistry, where electrons orchestrate a symphony of transformations, electrolytic cells emerge as captivating arenas where chemical energy is harnessed to drive non-spontaneous chemical reactions. This lesson will guide you through the art of sketching an electrolytic cell, empowering you to visualize the intricate arrangement of its components and comprehend their roles in this fascinating process.
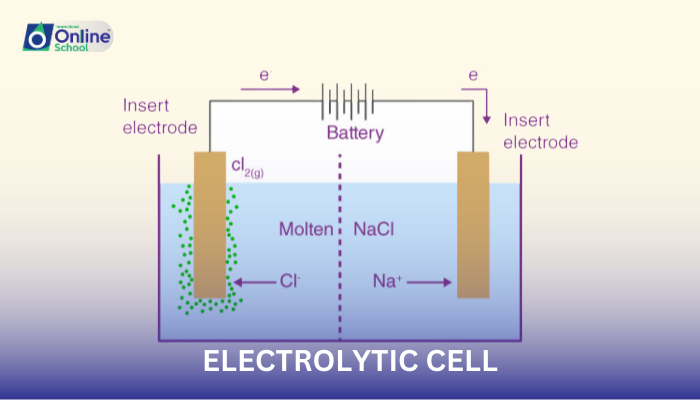
i. Sketching the Electrolytic Cell: A Step-by-Step Approach
Establish the Cell's Container: Draw a rectangular outline to represent the container that holds the electrolyte solution.
Introduce the Electrodes: Within the container, draw two parallel lines, representing the cathode and anode, the essential electrodes where redox reactions occur. The cathode is typically labeled as 'C-', while the anode is labeled as 'A+'.
Position the Salt Bridge: Connect the anode and cathode compartments with a wavy line representing the salt bridge, a porous barrier that allows ion flow between the electrolyte solutions.
Label the Electrolyte Solutions: Indicate the electrolyte solutions in each compartment, typically denoted as 'electrolyte 1' and 'electrolyte 2'.
External Energy Source: Outside the cell, draw an external electrical energy source, such as a battery, connected to the electrodes. This source provides the energy to drive the non-spontaneous chemical reaction.
ii. Significance of Electrolytic Cell Components
Each component of an electrolytic cell plays a crucial role in the process:
Cathode: The site of reduction, where electrons are gained and a species decreases its oxidation state.
Anode: The site of oxidation, where electrons are lost and a species increases its oxidation state.
Electrolyte: A conductive solution containing ions that facilitate the movement of electrons between the electrodes.
Salt bridge: A porous barrier that maintains electrical neutrality by allowing ion flow between the electrolyte solutions.
External electrical energy source: Provides the energy to drive the non-spontaneous chemical reaction.
Sketching an electrolytic cell provides a valuable tool for visualizing the components and their arrangement, enhancing understanding of this fascinating process. By accurately representing the cathode, anode, electrolyte, salt bridge, and external energy source, we gain insights into the intricate dance of electrons that drives non-spontaneous chemical reactions in electrolytic cells.